|
ACUTE ISCHAEMIC STROKE:
MANAGEMENT
|
Beneficial
|
- Aspirin
A systematic review of "eight RCTs comparing antiplatelet treatment
started within 14 days of the stroke versus placebo in 41,325 people
with definite or presumed ischaemic stroke was identified. 98% of the
data in the systematic review came from two large RCTs testing aspirin
160 to 300 mg daily started within 48 hours of stroke onset. Most patients
had an ischaemic stroke confirmed by CT scan before randomisation, but
those who were conscious could be randomised before CT scan if the stroke
was very likely to be ischaemic on clinical grounds. Treatment duration
varied from 10 to 28 days. Aspirin started within the first 48 hours
of acute ischaemic stroke reduced death and dependency at follow up
(RRR 3%, 95% CI 1% to 5%; NNT=77, 95% CI 43 to 333)."
{Source: Gubitz G, Sandercock P. Stroke: Management. Clinical Evidence
1999;2:104-12}
|
|
Trade off between benefits and
harms
- Thrombolysis
A Cochrane systematic review of 17 RCTs with 5,216 highly
selected patients was identified. All trials included a CT or magnetic
resonance scan to exclude intracranial haemorrhagic or other non-stroke
disorders. The trials tested urokinase, streptokinase, recombinant tissue
plasminogen activator or recombinant pro-urokinase.
- Thrombolysis reduced the risk of
being dead or dependent at the end of the studies (ARR 4.2%, 95%
CI 1.2% to 7.2%; RRR 7%, 95% CI 3% to 12%; NNT=24, 95% CI 14 to
83).
- Thrombolytic therapy administered
up to six hours after ischaemic stroke significantly reduced the
proportion of patients who were dead or dependent at the end of
follow up (OR 0.83, 95% CI 0.73 to 0.94).
For patients treated within three hours of stroke, thrombolytic
therapy appeared to be more effective in reducing death or dependency
(OR 0.58, 95% CI 0.46 to 0.74) with less adverse effect on death
(OR 1.11, 95% CI 0.84 to 1.47).
- Thrombolysis increased fatal intracranial
haemorrhage compared with placebo (ARI 4.4%, 95% CI 3.4% to 5.4%;
RRI 396%, 95% CI 220% to 668%; NNH=23, 95% CI 19 to 29).
- Thrombolytic treatment increased
the risk of death by the end of the follow up period compared with
placebo (ARI 3.3%, 95% CI 1.2% to 5.4%; RRI 23%, 95% CI 10% to 28%;
NNH=30, 95% CI 19 to 83). This excess of deaths was offset by fewer
people being alive but dependent six months after stroke onset.
The net effect was a reduction in the number of people who were
dead or dependent.
(Source: Wardlaw JM, del Zoppo G,
Yamaguchi T. Thrombolysis for acute ischaemic stroke. In: The Cochrane
Library (Online), Issue 2, 2000. Oxford: Update Software. [Last amendment:
21 Feb, 2000.])
|
|
Likely to be ineffective
or harmful
- Immediate systematic anticoagulation
A Cochrane systematic reviews of 21 RCTs involving 23,427 patients comparing
standard unfractionated heparin, low-molecular-weight heparins, heparinoids,
anticoagulants or thrombin inhibitors versus control was identified.
- No significant difference in the
proportion of people dead or dependent in the treatment or control
groups at the end of follow up (3 to 6 months after stroke, ARR
0.4%, 95% CI -0.9% to +1.7%; RRR 0%, 95% CI -2% to +3%). Majority
of the patient data (90%) came from a single trial evaluating unfractionated
heparin [IST 1997]. However, data from trials
of low molecular weight heparin [FISS 1995,
FISS-bis 1998] (OR=0.85, 95% CI 0.66 to
1.10), and heparinoid [TOAST 1999] (OR=0.92,
95% CI 0.72 to 1.19), also showed no clear evidence of benefit.
[Editorial note: FISS and
FISS-bis showed conflicting results; FISS study revealed that high
dose fraxiparine significantly lowered the risk of death or dependency
at 6 months (45%) compared to placebo (65%; p=0.005) whereas no evidence
of benefits were seen in the FISS-bis study.]
- Reduced the risk of deep vein thrombosis
(ARR 29%, 95% CI 24% to 35%; RRR 64%, 95% CI 54% to 71%; NNT=3,
95% CI 2 to 4).
- Reduced the development of symptomatic
pulmonary embolism (ARR 0.3%, 95% CI 0.1% to 0.6%; RRR 38%, 95%
CI 16% to 54%; NNT=333, 95% CI 167 to 1000).
- Increased intracranial haemorrhages
within 14 days of starting treatment (ARI 0.93%, 95% CI 0.68% to
1.18%; RRI 163%, 95% CI 95% to 255%; NNH=108, 95% CI 85 to 147).
There is a dose dependent increase in both intra- and extracranial
haemorrhage, although low dose regimens decrease deep vein thrombosis
and pulmonary embolus (which can be treated with aspirin or compression
stockings).
The reviewers concluded that "immediate
anticoagulant therapy in patients with acute ischaemic stroke is not
associated with net short- or long term benefit. The data from this
review do not support the routine use of any type of anticoagulant
in acute ischaemic stroke."
(Sources: Gubitz
G, Counsell C, Sandercock P, Signorini D. Anticoagulants for acute
ischaemic stroke. In: The Cochrane Library (Online), Issue 2, 2000.
Oxford: Update Software; International Stroke Trial Collaborative
Group. The International Stroke Trial (IST): a randomised
trial of aspirin, subcutaneous heparin, both, or neither among 19,435
patients with acute ischaemic stroke. Lancet 1997;349(9065):1569-81;
(FISS) Kay R, Wong KS, Yu YL, Chan YW, Tsoi TH,
Ahujai AT et al. Low-molecular-weight heparin for the treatment of
acute ischaemic stroke. N Eng J Med 1995 Dec 14;333(24):1588-93;
Hommel M, for the FISS-bis Investigator Group. Fraxiparine in
Ischaemic Stroke Study (FISS-bis) Cerebrovasc Dis 1998;8(suppl 4)
19; Adams HP, Bendixen BH, Leira E, Chang KC,
Davis PH, Woolson RF et al. Antithrombotic treatment of ischemic stroke
among patients with occlusion or severe stenosis of the internal carotid
artery: A report of the Trial of Org 10172 in Acute Stroke Treatment
(TOAST). Neurology 1999 Jul 13;53(1):122-5.}
|
- Acute reduction in blood pressure
- A Cochrane systematic review of
3 RCTs comparing blood pressure lowering treatment versus placebo
in 113 patients with acute stroke found insufficient evidence to
evaluate the effect of altering blood pressure after stroke.
(Source: Blood pressure in Acute
Stroke Collaboration (BASC). Interventions for deliberately altering
blood pressure in acute stroke. In: The Cochrane Library (Online),
Issue 2, 2000. Oxford: Update Software.)
|
|
|
|
|
|
|
|
|
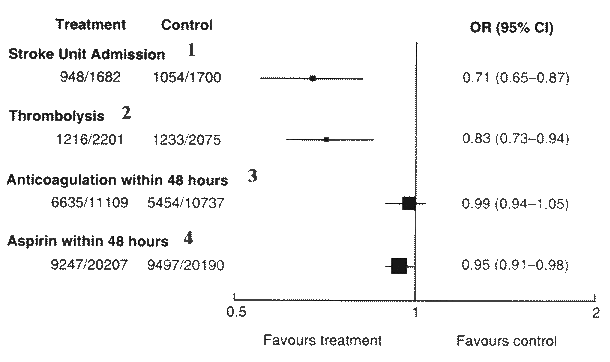
Fig.1.
The proportional effects of different interventions on 'death
or dependency' at the end of scheduled follow up: results of systematic
reviews. The data refer only to benefits and not to harms.
|
|
Fig.2.
Effect of thrombolysis on death and dependency at end of trial
follow up: results of a systematic review of RCTs 2.
|
|
| (Source: Gubitz G, Sandercock
P. Stroke: Management. Clinical Evidence 1999;2:104-12) |
| |
| References |
|
1.
|
Stroke Unit Trialists' Collaboration.
Organised inpatient (stroke unit) care for stroke. In: The Cochrane
Library (Online), Issue 2, 2000. Oxford: Update Software.
|
|
2.
|
Wardlaw
JM, del Zoppo G, Yamaguchi T. Thrombolysis for acute ischaemic stroke.
In: The Cochrane Library (Online), Issue 2, 2000. Oxford: Update Software.
|
|
3.
|
CAST (Chinese
Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled
trial of early aspirin use in 20,000 patients with acute ischaemic
stroke. Lancet 1997 June 7;349(9066):1641-9.
|
|
4.
|
International
Stroke Trial Collaborative Group. The International Stroke Trial (IST):
a randomised trial of aspirin, subcutaneous heparin, both, or neither
among 19,435 patients with acute ischaemic stroke. Lancet 1997 May
31;349(9065):1569-81.
|
|
5.
|
Morris
AD, Ritchie C, Grosset DG, Adams FG, Lees KR. A pilot study of streptokinase
for acute cerebral infarction. QJM 1995 Oct;88(10):727-31.
|
|
6.
|
The Multicenter
Acute Stroke Trial-Italy (MAST-I) Group. Randomised controlled trial
of streptokinase, aspirin, and combination of both in treatment of
acute ischaemic stroke. Lancet 1995 Dec 9;346(8989):1509-14.
|
|
7.
|
(MAST-E) The Multicenter Acute
Stroke Trial-Europe Study Group: Thrombolytic therapy with streptokinase
in acute ischemic stroke. N Engl J Med 1996 July 18;335(3):145-50.
|
|
8.
|
The Australian
Streptokinase (ASK) Trial Study Group. Streptokinase for acute ischemic
stroke with relationship to time of administration. JAMA 1996 Sept
25;276(12):961-6.
|
|
9.
|
Mori E,
Yoneda Y, Tabuchi M, Yoshida T, Ohkawa S, Ohsumi Y et al. Intravenous
recombinant tissue plasminogen activator in acute artery territory
stroke. Neurology 1992 Jan;42:976-82.
|
|
10
|
The European
Cooperative Acute Stroke Study (ECASS). Intravenous thrombolysis with
recombinant tissue plasminogen activator for acute hemispheric stroke.
JAMA 1995 Oct 5;274(13):1017-25.
|
|
11
|
The National
Institute of Neurological Disorders and Stroke rt-PA Stroke Study.
Tissue plasminogen activator for acute ischemic stroke. N Engl J Med
1995 Dec 14;333(24):1581-7.
|
|
| Additional information
and comments relative to this publication are welcome, and should
be addressed to Dr SP Lim at splim@ha.org.hk.
Reprint of this publication for the purpose of research or further
study is granted without prior permission from the Hospital Authority.
|
8 Feb 2000
|

